Zinc Pyrithione
| CAS No. | 13463-41-7 | Cat. No. | BCP13412 |
| Name | Zinc Pyrithione | ||
| Synonyms | |||
| Formula | C10H8N2O2S2Zn | M. Wt | 317.7 |
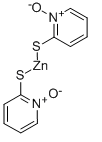
| Description | Zinc pyrithione is considered as a coordination complex of zinc. The pyrithione ligands, which are formally monoanions, are chelated to Zn 2+ via oxygen and sulfur centers. In the crystalline state, zinc pyrithione exists as a centrosymmetric dimer, where each zinc is bonded to two sulfur and three oxygen centers. In solution, however, the dimers dissociate via scission of one Zn-O bond. Zinc pyrithione, which is a dimer but is probably biologically active as a monomer, induces plasma membrane depolarization with half-maximal effect (K1/2) of about 0.3 mM [1]. Zinc pyrithione is an unusual synthetic potentiator that potently activates both heterologous and native M channels by inducing channel opening at the resting potential . Zinc pyrithione rapidly accumulated in the tissues of the exposed mussels, proportionately to both exposure concentration and time. Even though the 7-d median lethal concentration (LC50)?= 8.27μM established here appears high with respect to reported ZnPT enviro | ||
| Pathways | Ion Channel/Membrane Transporter | ||
| Targets | Proton Pump | ||
Structure
Part data of this page collected from the open network resources, so Biochempartner can not guarantee its accuracy.
For product details of different batches, please contact our Customer
- Service & Tech Support:orders@biochempartner.com
- Website:www.biochempartner.com
Products are for research use only and not for human use. We do not sell to patients.
