Isavuconazonium
| CAS No. | 742049-41-8 | Cat. No. | BCP23773 |
| Name | Isavuconazonium | ||
| Synonyms | BAL8557; BAL 8557; BAL-8557; | ||
| Formula | C35H35F2N8O5S+ | M. Wt | 717.77 |
| Description | Isavuconazonium is a second-generation triazole antifungal approved on March 6, 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis, marketed by Astellas under the brand Cresemba. It is the prodrug form of isavuconazole, the active moiety, and it is available in oral and parenteral formulations. Due to low solubility in water of isavuconazole on its own, the isovuconazonium formulation is favorable as it has high solubility in water and allows for intravenous administration. This formulation also avoids the use of a cyclodextrin vehicle for solubilization required for intravenous administration of other antifungals such as voriconazole and posaconazole, eliminating concerns of nephrotoxicity associated with cyclodextrin. | ||
| Pathways | Microbiology/Virology | ||
| Targets | Antifungal | ||
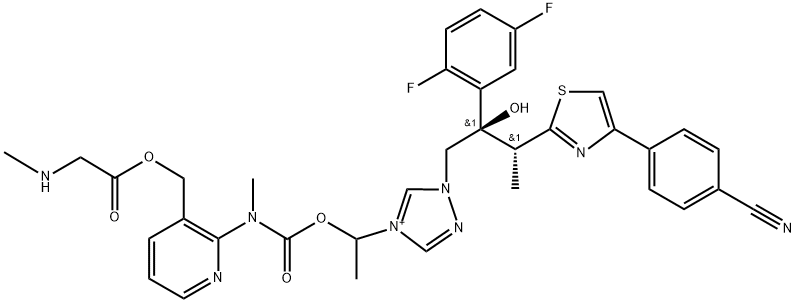
Structure
Part data of this page collected from the open network resources, so Biochempartner can not guarantee its accuracy.
For product details of different batches, please contact our Customer
- Service & Tech Support:orders@biochempartner.com
- Website:www.biochempartner.com
Products are for research use only and not for human use. We do not sell to patients.
