Brivaracetam
| CAS No. | 357336-20-0 | Cat. No. | BCP14251 |
| Name | Brivaracetam | ||
| Synonyms | UCB 34714;UCB-34714;UCB34714; Briviact; | ||
| Formula | C11H20N2O2 | M. Wt | 212.29 |
| Description | Brivaracetam has been tested in a comprehensive safety pharmacology, toxicology, developmental toxicology, and genotoxicity program. It is of low acute toxicity, target organ for toxic effects is the hepatobiliary tract. Carcinogenicity studies are ongoing. Human pharmacology studies have shown that brivaracetam has a half-life of 8 h and nearly complete bioavailability . CSD episodes were regularly elicited on slices upon delivery of calibrated KCl drops and were recorded via two micropipette electrodes. After control CSDs, the drug was added to the perfusion and five subsequent CSDs were elicited during drug perfusion. Effects were assessed via CSD amplitude (Ampl) and duration at half-amplitude (D(1/2)). BRV, 10 and 32 microM reduced the Ampl and transiently the D(1/2). Levetiracetam, 32 and 100 microM had no effect on either Ampl or D(1/2) . Brivaracetam bound selectively with 20 fold higher affinity than levetiracetam to SV2A . BRV produced a concentration-dependent inhibition of | ||
| Pathways | Ion Channel/Membrane Transporter | ||
| Targets | Sodium Channel | ||
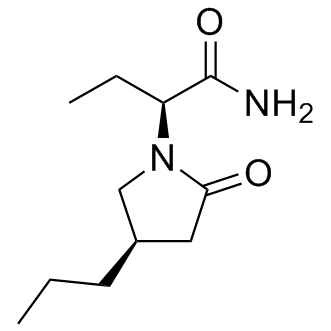
Structure
Part data of this page collected from the open network resources, so Biochempartner can not guarantee its accuracy.
For product details of different batches, please contact our Customer
- Service & Tech Support:orders@biochempartner.com
- Website:www.biochempartner.com
Products are for research use only and not for human use. We do not sell to patients.
