
Doxifluridine
 Data Sheet
For research use only. Not for human use.
Data Sheet
For research use only. Not for human use.
* Required Fields.
Please complete the form below and you will get the price list in 1 minute.
* Required Fields.
Please complete the form below and we will contact you shortly.
* Required Fields.
Please complete the form below and we will contact you shortly.

| CAS No. | 3094-09-5 | Cat. No. | BCP12582 |
| Name | Doxifluridine | ||
| Synonyms | Flutron;Furtulon;Ro 21-9738;5-Fluoro-5(acute)-deoxyuridine; 5(acute)-DFUR; | ||
| SMILES | |||
| Chemical Name | |||
| Formula | C9H11FN2O5 | M. Wt | 246.19 |
| Purity | 98% | Storage | Store at 4-8°C |
| Description | Doxifluridine is a fluoropyrimidine derivative and oral prodrug of the antineoplastic agent 5-fluorouracil (5-FU) with antitumor activity. Doxifluridine, designed to circumvent the rapid degradation of 5-FU by dihydropyrimidine dehydrogenase in the gut wall, is converted into 5-FU in the presence of pyrimidine nucleoside phosphorylase. 5-FU interferes with DNA synthesis and subsequent cell division by reducing normal thymidine production and interferes with RNA transcription by competing with uridine triphosphate for incorporation into the RNA strand. in vitro: 5'-DFUR's metabolic product(N3-Me-5'-dFUR) was found to be non-toxic in all the cell growth experiments performed. The absence of cytotoxicity could be explained by the observation that the metabolite was not recognized as a substrate by thymidine phosphorilase, the enzyme responsible for 5-fluorouracil (5-FU) release from doxifluridine, as ascertained by high-performance liquid chromatography/ultraviolet (HPLC-UV) analysis of t | ||
Recommend Products
More >
-
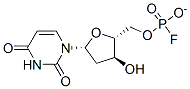
Fluorodeoxyuridylate
Cat. No.:BCP31154
No.:134-46-3
Product Details -

Capecitabine
Cat. No.:BCP29814
No.:158798-73-3
Product Details -

Spongouridine
Cat. No.:BCP29295
No.:3083-77-0
Product Details -
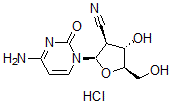
CNDAC HCl salt
Cat. No.:BCP19489
No.:134665-72-8
Product Details -
-
-
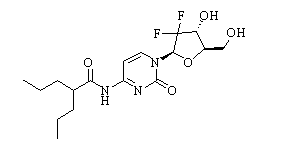
-
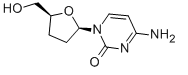
-
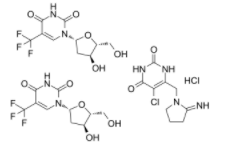
-
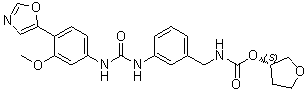
MeriMepodib
Cat. No.:BCP28336
No.:198821-22-6
Product Details -
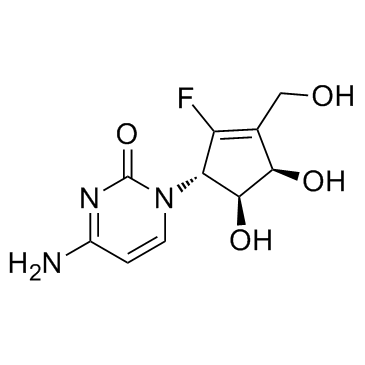
-
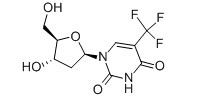
-
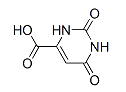
-
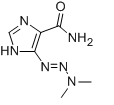
-

-
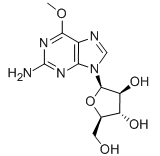
Nelarabine
Cat. No.:BCP02093
No.:121032-29-9
Product Details -
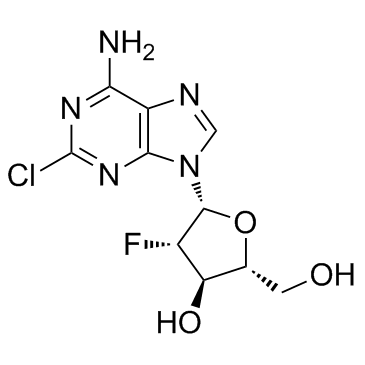
Clofarabine
Cat. No.:BCP23422
No.:123318-82-1
Product Details -
-
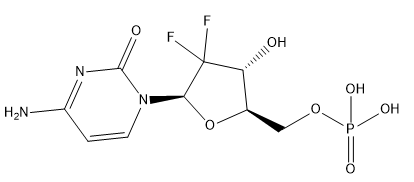
Gemcitabine Monophosphate
Cat. No.:BCP21656
No.:116371-67-6
Product Details -
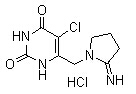
Tipiracil HCl
Cat. No.:BCP06245
No.:183204-72-0
Product Details

Tags:Doxifluridine supplier,Doxifluridine purchase,Doxifluridine manufacturer,Doxifluridine distributor,Doxifluridine cost,Doxifluridine buy,Doxifluridine for sale




